

![]()
Medi-Cal NDC Update August 25, 2008

August 15, 2008
Beginning September 1, 2008
Beginning September 1, 2008, providers are encouraged to begin using the National Drug Code (NDC) for physician-administered drugs, in conjunction with the customary Healthcare Common Procedure Coding System (HCPCS) Level I, II or III code, on all Medi-Cal claims.
Claims submitted for dates of service from September 1, 2008 through March 31, 2009 without an NDC will not be denied.
Claims with dates of service on or after April 1, 2009 that do not meet the NDC reporting requirements to include a valid NDC paired with a HCPCS code, will result in claims being denied.
The Deficit Reduction Act of 2005 (DRA) requires all state Medicaid agencies to collect rebates from drug manufacturers for physician-administered or dispensed drugs. Only those products manufactured by companies participating in the federal Medicaid rebate program are reimbursable under Medi-Cal. A list of manufacturers participating in the rebate program, which changes periodically, is available in the Part 2 Medi-Cal pharmacy manual under Drugs: Contract Drugs List Part 5 – Authorized Manufacturer Labeler Codes.
National Drug Code Description
The NDC is a number that identifies a specific drug. The NDC number consists of 11 digits in a 5-4-2 format. NDCs printed on packages often have fewer than 11 digits, with hyphens (-) separating the number into three segments. A complete 11-digit number must have five digits in the first segment, four digits in the second segment, and two digits in the last segment. The first five digits of an NDC identify the manufacturer of the drug and are assigned by the Food and Drug Administration (FDA). The remaining digits are assigned by the manufacturer and identify the specific product and package size. Leading zeros are added wherever they are needed to complete a segment with the correct number of digits.
|
|
Example: 5-4-2 Format |
||
|
|
Package Number |
Zero Fill |
11-digit NDC |
|
|
1234-1234-12 |
(01234-1234-12) |
01234123412 |
|
|
12345-123-12 |
(12345-0123-12) |
12345012312 |
|
|
2-22-2 |
(00002-0022-02) |
00002002202 |
The NDC is found on the drug container (vial, bottle or tube). The NDC submitted to Medi-Cal must be the actual NDC number on the package or container from which the medication was administered. Providers should not bill for one manufacturer’s product and dispense another. It is considered to be a fraudulent billing practice to bill using an NDC other than the one administered.
Physician-Administered Drugs
A physician-administered drug includes any covered outpatient drug provided or administered to a recipient, which is billed by a provider other than a pharmacy. Such providers would include, but not be limited to, physician offices, clinics and hospitals. A covered outpatient drug is broadly defined as a drug that may be dispensed only upon prescription and is approved for safety and effectiveness as a prescription drug under the Federal Food, Drug and Cosmetic Act. Physician-administered drugs are not restricted to injectable drugs only. Physician-administered drugs include any drug regardless of the method of administration.
Drug Identification Guidelines
There are three items to look for that will identify whether or not a product is a drug:
NDC – The vial or box that held the drug would have an NDC on it that will be used for claims.
Lot and Expiration Date – All drugs have both a lot number and expiration date on the vial or box.
Legend – This refers to statements such as, “Caution: Federal law prohibits dispensing without prescription,” “Rx only” or similar words. All prescription drugs have these types of statements.
Claims Processing
Claims will continue to be priced based on the HCPCS code, with the NDC and corresponding units being used for drug rebate processing. Medicare primary claims will also require NDCs with HCPCS codes.
Quantity Reporting
Reporting instructions apply to both paper claims and electronic transactions. At this time, Medi-Cal will use only the HCPCS quantities/units for payment and rebate purposes. Sometimes it may be necessary for providers to bill multiple NDCs for a single procedure code. This may happen when two different strengths of the same drug are needed in order to administer the appropriate dose. This will also be necessary when multiple vials of the same drug are used to administer the appropriate dose, and the vials are manufactured by different manufacturers. When a provider uses more than one NDC for a drug, the provider must include all NDCs on the claim. The quantity for each NDC must be reported separately by repeating the HCPCS code.
Paper Claims
CMS-1500 Claim Form
For paper claims submitted on the CMS-1500 claim form, the NDC is reported in the shaded area of Box 24A.
Box 24A (shaded area) – This area will have a combination of two values entered:
Bytes 1 and 2 will include the Product ID Qualifier. This qualifier identifies the type of number that is being provided, which is an NDC, with a qualifier of ‘N4’. Bytes 3 – 21 will consist of the entry of the appropriate number for the qualifier entered in the first two digits. The length of this additional information will vary dependent upon the type of number being provided (as identified by the previous 2-byte Product ID Qualifier).
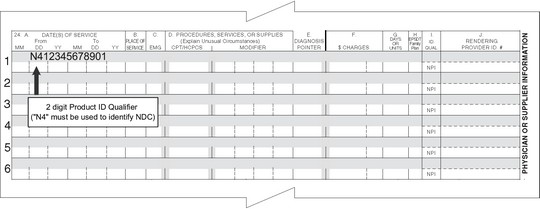
Example: N4 as the Product ID Qualifier, followed by the 11-digit NDC – N412345678901
Box 24D – The HCPCS code will continue to be entered in 24D, with the charges in Box 24F and units in Box 24G.
Box 24D (shaded area) – In this area, enter the NDC unit of measure (two positions) immediately followed by the numeric quantity administered to the patient, which is a full
10-digit number. The 10 digits consist of seven digits for the whole number, followed by the three-digit decimal portion of the number.
Note: The quantity field should be entered from left to right; do not enter a decimal. Valid Unit of Measurement Qualifiers are the following:
F2 = International Unit
GR = Gram
ML = Milliliter
UN = Unit
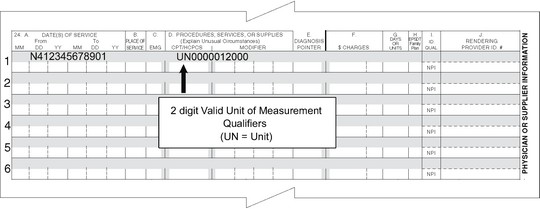
Examples: UN0000012000 for a quantity of 12 units, ML0000124540 for a quantity of 124.54 milliliters
UB-04 Claim Form
For paper claims submitted on the UB-04 claim form, the NDC is reported in the Description field (Box 43).
Box 43 – Enter the two-digit Product ID Qualifier ‘N4’ in the first two positions, immediately followed by the 11-digit NDC (no hyphens). Directly following the last digit of the NDC (no delimiter), enter the two-digit Unit of Measurement Qualifier as noted above. Immediately following the Unit of Measurement Qualifier, enter the nine-digit quantity. The nine digits consist of six digits for the whole number, followed by the three-digit decimal portion of the number.
Note: The Description field on the UB-04 form is 24 characters in length.
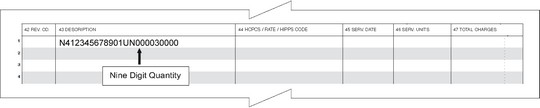
Example: Unit quantity of 30 for NDC 12345678901: N412345678901UN000030000
Box 44 – Using the HCPCS/RATE/HIPPS Code field, enter the five-character HCPCS code.
Box 46 – Using the Serv. Units field, enter the corresponding service units for the HCPCS reported.
Direct Data Entry
The Point of Service (POS) device and Internet Professional Claim Submission (IPCS) system have not been updated yet. In the interim, providers using these means of claims submission should continue to provide the HCPCS code only. These applications will be remediated and HCPCS/NDC pairing will be required beginning with dates of service on April 1, 2009 and after. Please watch for more detailed information in future Medi-Cal Updates.
HIPAA 837 Professional Transactions
For HIPAA-compliant ASC X12N 837 Professional electronic claim transactions, the HCPCS code is reported in Loop ID 2400.
Loop 2400:
Field SV1 – Enter HCPCS code.
Field SV101 – 3 – Enter the UD modifier if the drug was obtained under the 340B program.
The NDC is reported in Loop ID 2410.
Loop 2410:
Field CTP04 – Enter quantity.
Field CTP05 – Enter unit of measurement.
Field LIN02 – Enter qualifier ‘N4’.
Field LIN03 – Enter NDC without hyphens.
Examples: CTP****2*UN~ and LIN**N4*12345678901~
HIPAA 837 Institutional Transactions
For HIPAA-compliant ASC X12N 837 Institutional electronic claim transactions, the HCPCS code is reported in Loop ID 2400.
Loop 2400:
Field SV201 – Enter the national code.
Field SV202-1 – Enter qualifier ‘HC’.
Field SV202-2 – Enter the HCPCS code.
Field SV202-3 – Enter the UD modifier if you obtained the drug under the 340B program.
Field SV204 – Enter qualifier ‘UN’.
Field SV205 – Enter the quantity.
Example: SV2*250*HC*Jxxxx**UN*1~
Loop 2410:
Field LIN02 – Enter qualifier ‘N4’.
Field LIN03 – Enter NDC without hyphens.
Example: LIN**N4*12345678901~
Field CTP04 – Enter quantity.
Field CTP05 – Enter unit of measure.
Example: CTP****2*ML~
For more detailed information, please refer to the billing instructions for electronic claim transactions found in the 837 Transaction Companion Guides page.
Section 340B of the Public Health Service Act
Background Information
The 340B Drug Pricing Program resulted from enactment of Public Law 102-585, the Veterans Health Care Act of 1992, which is codified as Section 340B of the Public Health Service Act. Section 340B limits the cost of covered outpatient drugs to certain federal grantees, federally-qualified health center look-alikes and qualified disproportionate share hospitals. Significant savings on pharmaceuticals may be seen by those entities that participate in this program.
Section 340B of the Public Health Service Act provides that a manufacturer who sells covered outpatient drugs to eligible entities must sign a pharmaceutical pricing agreement with the Secretary of Health and Human Services, in which the manufacturer agrees to charge a price for covered outpatient drugs that will not exceed the average manufacturer price ("AMP") decreased by a rebate percentage. Section 340B also requires eligible entities to charge the Medicaid program no more than the actual acquisition cost of the drug plus the state allowed dispensing/administration fee, and to require the state Medicaid program to exclude these claims from the collection of rebates.
340B Outpatient Drugs – UD Modifier
In order for providers to identify 340B outpatient drugs that have been dispensed, the National Medicaid EDI Healthcare (NMEH) has recommended use of the ‘UD’ modifier. This will allow Medicaid to identify those claims that are from 340B entities and exclude them from the rebate collection process. The ‘UD’ modifier should be billed on the CMS-1500 & 837 Professional and the UB-04 & 837 Institutional claim forms, associated with the applicable HCPCS code and NDC, to properly identify 340B drugs. The ‘UD’ modifier is to be used only in this circumstance. All non-340B drugs are billed using the applicable HCPCS and NDC pair without a modifier.